Buprenorphine was originally approved by the FDA in 2002 to treat opioid addiction in medication-assisted treatment (MAT) programs. It is a partial opioid agonist that binds to opioid receptors to activate them in a similar way as opioids do. The medication works to alleviate the pain of opioid withdrawal and reduce drug cravings.
There are many different prescription medications that contain buprenorphine, two of which include Zubsolv and Suboxone.
What is Suboxone?
Suboxone was approved by the FDA to treat opioid dependence and addiction. It contains two medications: buprenorphine and naloxone. As a Schedule III controlled substance, it can only be prescribed by physicians who have completed specialized certifications to administer medications for medication-assisted treatment (MAT) and substance use disorders.[1]
Suboxone comes in the form of a sublingual film that is placed under the tongue or between the cheek and gums where it dissolves. The medication is available in four different strengths, all with a 4:1 ratio of buprenorphine to naloxone:
- 2 mg buprenorphine/0.5 mg naloxone
- 4 mg buprenorphine/1 mg naloxone
- 8 mg buprenorphine/2 mg naloxone
- 12 mg buprenorphine/3 mg naloxone
Suboxone is intended only for patients who have agreed to participate in a drug and alcohol treatment program. This is because the medication is most effective when combined with behavioral therapy and peer support.
Suboxone Side Effects
Like all medications, Suboxone may cause side effects. The most common side effects of Suboxone are:
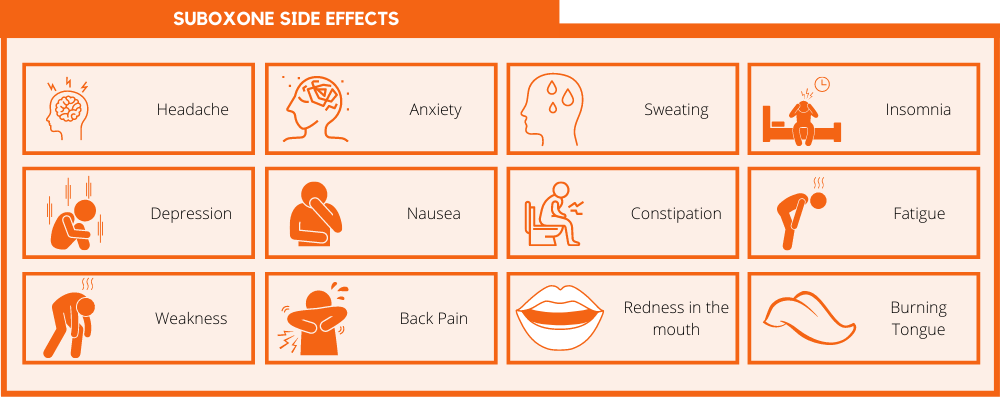
- Headache
- Anxiety
- Sweating
- Insomnia
- Depression
- Nausea
- Constipation
- Fatigue
- Weakness
- Back pain
- Redness in the mouth
- Burning tongue
What is Zubsolv?
Zubsolv is a brand-name medication that contains buprenorphine and naloxone. It was approved by the FDA one year after Suboxone for the treatment of opioid dependence and addiction.
Zubsolv comes in the form of a sublingual tablet that dissolves when placed under the tongue. Being similar to Suboxone in the way that it is a Schedule III controlled substance, this medication can only be prescribed by doctors who have completed special training and received certification from the federal government.
Zubsolv is available in six different strengths, each with a different shape pill and a 4:1 ratio of buprenorphine and naloxone:
- 0.7 mg of buprenorphine/0.18 mg of naloxone (oval shape)
- 1.4 mg of buprenorphine/0.36 mg of naloxone (triangle shape)
- 2.9 mg of buprenorphine/0.71 mg of naloxone (D shape)
- 5.7 mg of buprenorphine/1.4 mg of naloxone (round shape)
- 8.6 mg of buprenorphine/2.1 mg of naloxone (diamond shape)
- 11.4 mg of buprenorphine/2.9 mg of naloxone (capsule shape)
Like Suboxone, Zubsolv should always be used in combination with a comprehensive addiction treatment program.
Zubsolv Side Effects
Zubsolv contains the same ingredients as Suboxone, so it produces many of the same side effects. These include:
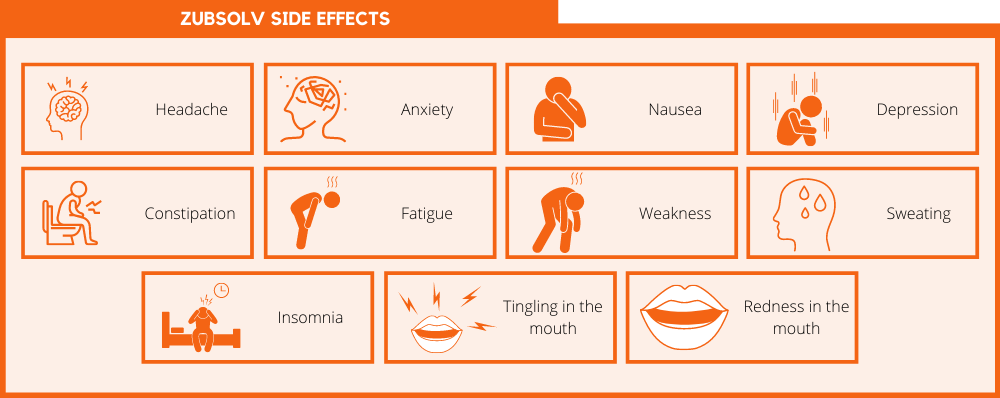
- Headache
- Anxiety
- Nausea
- Depression
- Constipation
- Fatigue
- Weakness
- Sweating
- Insomnia
- Tingling in the mouth
- Redness in the mouth
Differences Between Zubsolv and Suboxone
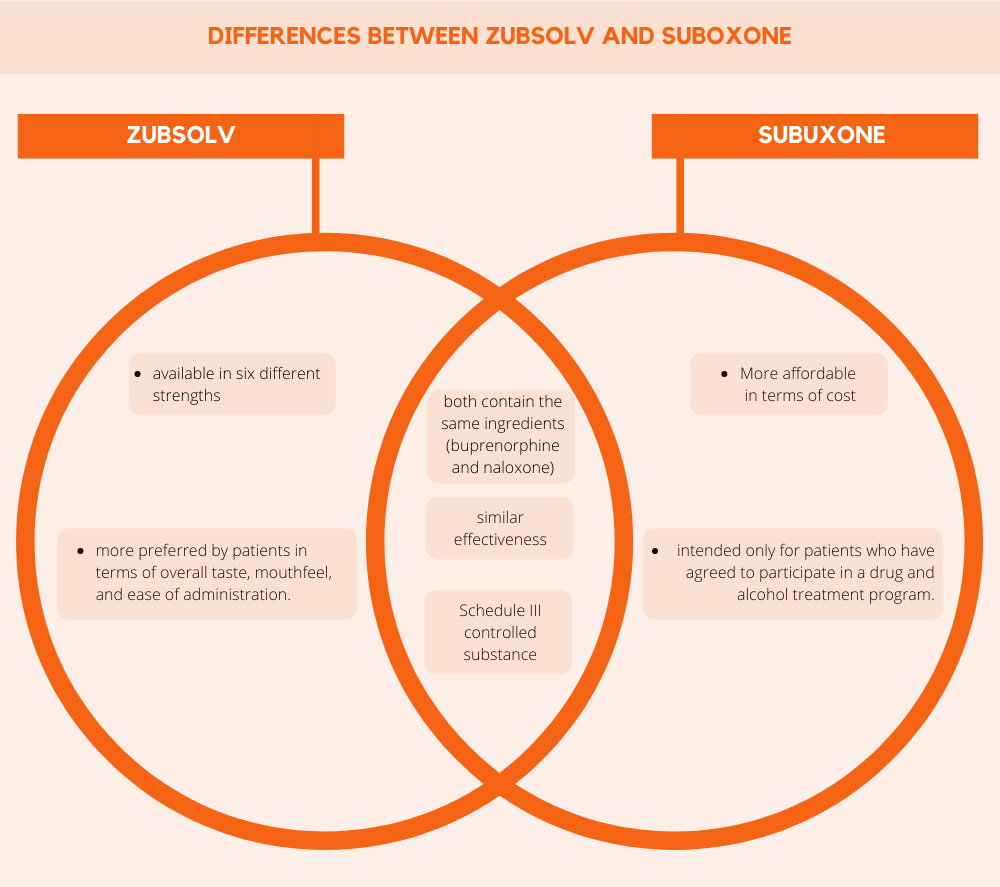
Zubsolv and Suboxone both contain the same ingredients (buprenorphine and naloxone), are used to treat opioid use disorder, and have similar side effects. In addition, clinical studies have found that both medications have similar effectiveness overall.[3] While both drugs are thought to be the same in terms of effectiveness, there are some benefits of each.
The primary advantage of taking Zubsolv is that it is available in six different strengths, each of which is different from the traditional dosages offered by competitors like Suboxone and Subutex. By having more options, and options that are not available in other buprenorphine-containing medications, physicians are able to offer patients doses that work best for their bodies.
In the same study that compared Suboxone and Zubsolv in terms of efficiency, more patients preferred Zubsolv in terms of overall taste, mouthfeel, and ease of administration.[3] Suboxone can leave a person’s mouth feeling tingly with a strong, lingering, and unpleasant taste. Far fewer patients reported this with Zubsolv.
In terms of cost, however, some people find that Suboxone is more affordable. This is because there are numerous generic forms of Suboxone available on the market that are cheaper than the brand-name version. As of February 2021, there are no generic forms of Zubsolv available.
In the end, the American Society of Addiction Medicine (ASAM) doesn’t recommend either of these medications over the other. Rather, they suggest you consult with your doctor to discuss the pros and cons of each medication to determine which one is right for you.[4]
Lastly, both medications should be used in combination with an addiction treatment program that utilizes counseling and peer support as neither of these drugs are intended to be the sole treatment for a drug problem.
Find Help Today
When combined with an individualized treatment plan and evidence-based therapies, Suboxone and Zubsolv can help you overcome your addiction to opioids.
PAX Memphis Recovery Center offers an affordable Suboxone treatment program in Memphis, TN. All of our Suboxone patients actively participate in one of our drug rehab programs involving support groups, behavioral therapy, and individual counseling.
If you or a loved one are interested in learning more about medication-assisted treatment (MAT) or any of our addiction treatment programs, pick up the phone and call today.
References:
- https://opioidpreventionandtreatment.ucsf.edu/sites/g/files/tkssra506/f/wysiwyg/BCMJ_Vol60_No8_suboxone_guide.pdf
- https://journals.lww.com/journaladdictionmedicine/fulltext/2016/04000/Efficacy_of_Buprenorphine_Naloxone_Rapidly.8.aspx
- https://www.zubsolv.com/healthcareprofessionals/about-zubsolv/study-006/
Medically Reviewed: September 25, 2019

All of the information on this page has been reviewed and verified by a certified addiction professional.